Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
- Prognostic Value of Triglyceride and Glucose Index for Incident Type 2 Diabetes beyond Metabolic Health and Obesity
- Hwi Seung Kim, Jiwoo Lee, Yun Kyung Cho, Eun Hee Kim, Min Jung Lee, Hong-Kyu Kim, Joong-Yeol Park, Woo Je Lee, Chang Hee Jung
- Endocrinol Metab. 2021;36(5):1042-1054. Published online October 21, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1184
- 5,286 View
- 133 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Metabolically healthy obese (MHO) phenotype is metabolically heterogeneous in terms of type 2 diabetes (T2D). Previously, the triglyceride and glucose (TyG) index has been considered for identifying metabolic health and future risk of T2D. This study aimed to evaluate the risk of incident T2D according to obesity status and metabolic health, categorized by four different criteria and the TyG index.
Methods
The study included 39,418 Koreans without T2D at baseline. The risk of T2D was evaluated based on four different definitions of metabolic health and obesity status and according to the baseline TyG index within each metabolic health and obesity group.
Results
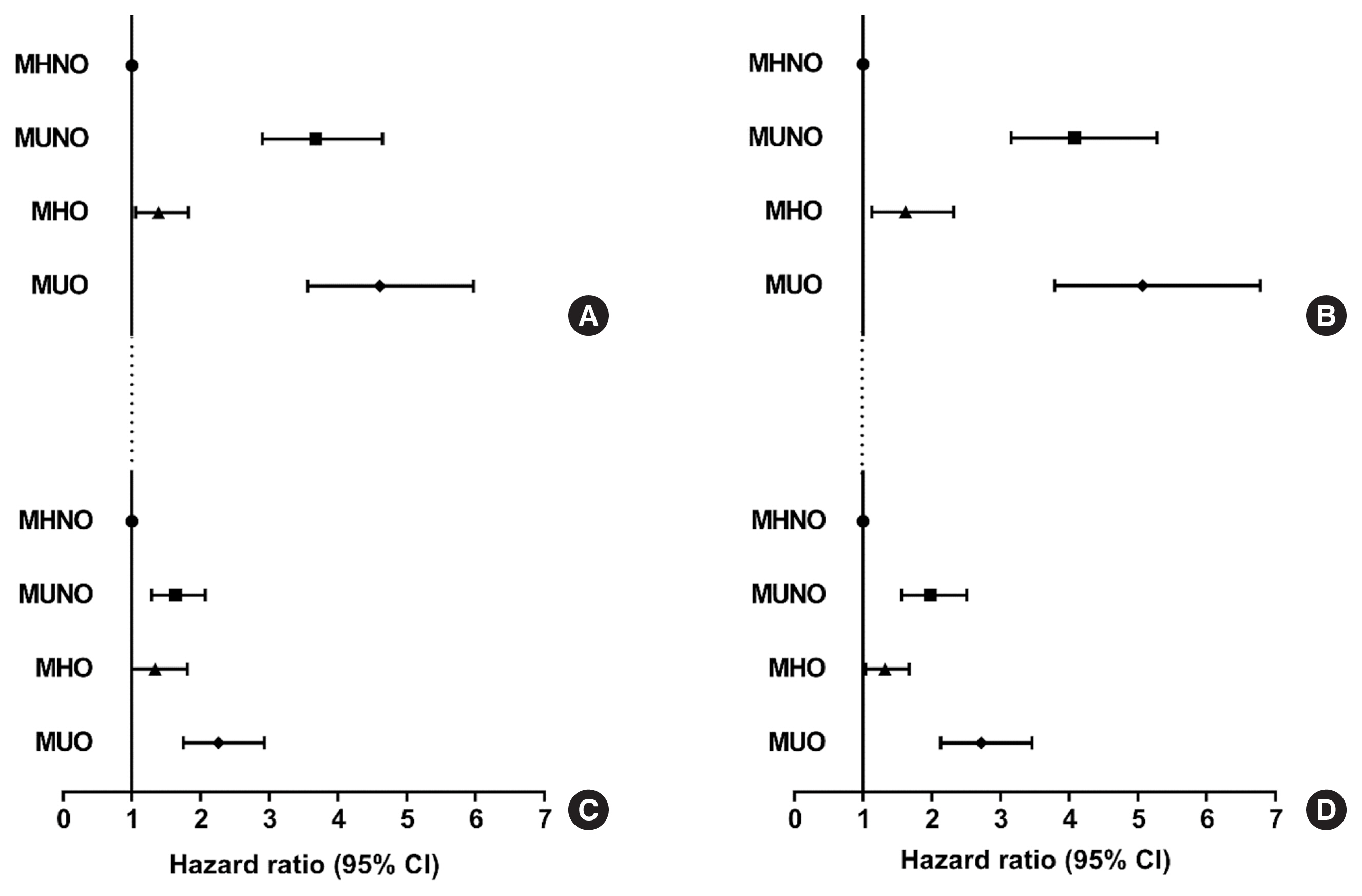
During the median follow-up at 38.1 months, 726 individuals developed T2D. Compared with the metabolically healthy non-obese (MHNO) group with low TyG index, the MHO group with high TyG index showed increased risk of T2D in all four definitions of metabolic health with multivariate-adjusted hazard ratios of 2.57 (95% confidence interval [CI], 1.76 to 3.75), 3.72 (95% CI, 2.15 to 6.43), 4.13 (95% CI, 2.67 to 6.38), and 3.05 (95% CI, 2.24 to 4.15), when defined by Adult Treatment Panel III, Wildman, Karelis, and homeostasis model assessment (HOMA) criteria, respectively.
Conclusion
MHO subjects with high TyG index were at an increased risk of developing T2D compared with MHNO subjects, regardless of the definition of metabolic health. TyG index may serve as an additional factor for predicting the individual risk of incident T2D in MHO subjects.

- Effects of Incretin-Based Therapies on Diabetic Microvascular Complications
- Yu Mi Kang, Chang Hee Jung
- Endocrinol Metab. 2017;32(3):316-325. Published online September 18, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.3.316
- 4,573 View
- 55 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The morbidity and mortality associated with diabetic complications impose a huge socioeconomic burden worldwide. Therefore, the ultimate goal of managing diabetes mellitus (DM) is to lower the risk of macrovascular complications and highly morbid microvascular complications such as diabetic nephropathy (DN) and diabetic retinopathy (DR). Potential benefits of incretin-based therapies such as glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and dipeptidyl peptidase-4 (DPP-4) inhibitors on the diabetic macrovascular complications have been recently suggested, owing to their pleiotropic effects on multiple organ systems. However, studies primarily investigating the role of these therapies in diabetic microvascular complications are rare. Nevertheless, preclinical and limited clinical data suggest the potential protective effect of incretin-based agents against DN and DR via their anti-inflammatory, antioxidative, and antiapoptotic properties. Evidence also suggests that these incretin-dependent and independent beneficial effects are not necessarily associated with the glucose-lowering properties of GLP-1 RAs and DPP-4 inhibitors. Hence, in this review, we revisit the preclinical and clinical evidence of incretin-based therapy for DR and DN, the two most common, morbid complications in individuals with DM. In addition, the review discusses a few recent studies raising concerns of aggravating DR with the use of incretin-based therapies.
-
Citations
Citations to this article as recorded by- Efficacy and Safety of the Utilization of Dipeptidyl Peptidase IV Inhibitors in Diabetic Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials
Moeber Mahzari, Muhannad Alqirnas, Moustafa Alhamadh, Faisal Alrasheed, Abdulrahman Alhabeeb, Wedad Al Madani, Hussain Aldera
Diabetes, Metabolic Syndrome and Obesity.2024; Volume 17: 1425. CrossRef - Anti-Inflammatory Effects of GLP-1R Activation in the Retina
Alessandra Puddu, Davide Maggi
International Journal of Molecular Sciences.2022; 23(20): 12428. CrossRef - Diabetes and Its Complications: Therapies Available, Anticipated and Aspired
Anu Grover, Komal Sharma, Suresh Gautam, Srishti Gautam, Monica Gulati, Sachin Kumar Singh
Current Diabetes Reviews.2021; 17(4): 397. CrossRef - SGLT2 Inhibitors, GLP-1 Agonists, and DPP-4 Inhibitors in Diabetes and Microvascular Complications: A Review
Christopher El Mouhayyar, Ruba Riachy, Abir Bou Khalil, Asaad Eid, Sami Azar
International Journal of Endocrinology.2020; 2020: 1. CrossRef - Novel therapeutic agents for the treatment of diabetic kidney disease
Rachel E. Hartman, P.S.S. Rao, Mariann D. Churchwell, Susan J. Lewis
Expert Opinion on Investigational Drugs.2020; 29(11): 1277. CrossRef - Nationwide Trends in Pancreatitis and Pancreatic Cancer Risk Among Patients With Newly Diagnosed Type 2 Diabetes Receiving Dipeptidyl Peptidase 4 Inhibitors
Minyoung Lee, Jiyu Sun, Minkyung Han, Yongin Cho, Ji-Yeon Lee, Chung Mo Nam, Eun Seok Kang
Diabetes Care.2019; 42(11): 2057. CrossRef - Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Jae Hyun Bae, Sunhee Kim, Eun-Gee Park, Sin Gon Kim, Seokyung Hahn, Nam Hoon Kim
Endocrinology and Metabolism.2019; 34(1): 80. CrossRef - Serum adipocytokines are associated with microalbuminuria in patients with type 1 diabetes and incipient chronic complications
Tomislav Bulum, Marijana Vučić Lovrenčić, Martina Tomić, Sandra Vučković-Rebrina, Vinko Roso, Branko Kolarić, Vladimir Vuksan, Lea Duvnjak
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 496. CrossRef - Protective Effects of Incretin Against Age-Related Diseases
Di Zhang, Mingzhu Ma, Yueze Liu
Current Drug Delivery.2019; 16(9): 793. CrossRef - The role of dipeptidylpeptidase-4 inhibitors in management of cardiovascular disease in diabetes; focus on linagliptin
Annayya R. Aroor, Camila Manrique-Acevedo, Vincent G. DeMarco
Cardiovascular Diabetology.2018;[Epub] CrossRef
- Efficacy and Safety of the Utilization of Dipeptidyl Peptidase IV Inhibitors in Diabetic Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials

- Obesity and Metabolism
- Cardiovascular Effects of Glucagon-Like Peptide-1 Receptor Agonists
- Yu Mi Kang, Chang Hee Jung
- Endocrinol Metab. 2016;31(2):258-274. Published online April 25, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.258
- 6,374 View
- 92 Download
- 31 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Glucagon-like peptide-1 (GLP-1) is a member of the proglucagon incretin family, and GLP-1 receptor agonists (RAs) have been introduced as a new class of antidiabetic medications in the past decade. The benefits of GLP-1 RAs are derived from their pleiotropic effects, which include glucose-dependent insulin secretion, suppressed glucagon secretion, and reduced appetite. Moreover, GLP-1 RAs also exert beneficial roles on multiple organ systems in which the GLP-1 receptors exist, including the cardiovascular system. Cardiovascular effects of GLP-1 RAs have been of great interest since the burden from cardiovascular diseases (CVD) has been unbearably increasing in a diabetic population worldwide, despite strict glycemic control and advanced therapeutic techniques to treat CVD. Preclinical studies have already demonstrated the beneficial effects of GLP-1 on myocardium and vascular endothelium, and many clinical studies evaluating changes in surrogate markers of CVD have suggested potential benefits from the use of GLP-1 RAs. Data from numerous clinical trials primarily evaluating the antihyperglycemic effects of multiple GLP-1 RAs have also revealed that changes in most CVD risk markers reported as secondary outcomes have been in favor of GLP-1 RAs treatment. However, to date, there is only one randomized clinical trial of GLP-1 RAs (the ELIXA study) evaluating major cardiovascular events as their primary outcomes, and in this study, a neutral cardiovascular effect of lixisenatide was observed in high-risk diabetic subjects. Therefore, the results of ongoing CVD outcome trials with the use of GLP-1 RAs should be awaited to elucidate the translation of benefits previously seen in CVD risk marker studies into large clinical trials with primary cardiovascular outcomes.
-
Citations
Citations to this article as recorded by- Cardioprotective Actions of a Glucagon‐like Peptide‐1 Receptor Agonist on Hearts Donated After Circulatory Death
Sachiko Kadowaki, M. Ahsan Siraj, Weiden Chen, Jian Wang, Marlee Parker, Anita Nagy, Chun‐Po Steve Fan, Kyle Runeckles, Jing Li, Junko Kobayashi, Christoph Haller, Mansoor Husain, Osami Honjo
Journal of the American Heart Association.2023;[Epub] CrossRef - The role of dipeptidyl peptidase-IV in abdominal aortic aneurysm pathogenesis: A systematic review
Elisha Ngetich, Pierfrancesco Lapolla, Anirudh Chandrashekar, Ashok Handa, Regent Lee
Vascular Medicine.2022; 27(1): 77. CrossRef - Glucagon-like Peptide-1 Receptor Agonists in the Management of Type 2 Diabetes Mellitus and Obesity: The Impact of Pharmacological Properties and Genetic Factors
Jasna Klen, Vita Dolžan
International Journal of Molecular Sciences.2022; 23(7): 3451. CrossRef - Glucagon-like peptide-1 (GLP-1) receptor agonists and cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of double-blind, randomized, placebo-controlled clinical trials
Jing Qin, Li Song
BMC Endocrine Disorders.2022;[Epub] CrossRef - Role of G-protein coupled receptor (GPCRs)/(GPR-120) as an agonists in diabetic wound healing
Jagat Pal Yadav, Dinesh Kumar Patel, Prateek Pathak, Maria Grishina
Obesity Medicine.2022; 36: 100466. CrossRef - Protection against stroke with glucagon-like peptide-1 receptor agonists: a comprehensive review of potential mechanisms
Bruno Vergès, Victor Aboyans, Denis Angoulvant, Pierre Boutouyrie, Bertrand Cariou, Fabien Hyafil, Kamel Mohammedi, Pierre Amarenco
Cardiovascular Diabetology.2022;[Epub] CrossRef - Changing Fields-Diabetes Medications Invading the Cardiovascular Space
Lauren D. Breite, Mackenzie Steck, Brandon Tate Cutshall, Samarth P. Shah, Brandon E. Cave
Current Problems in Cardiology.2021; 46(3): 100736. CrossRef - PEGDA/HA mineralized hydrogel loaded with Exendin4 promotes bone regeneration in rat models with bone defects by inducing osteogenesis
Wei Liu, Xiaowei Jing, Zhiwen Xu, Chong Teng
Journal of Biomaterials Applications.2021; 35(10): 1337. CrossRef - Metabolite G-Protein Coupled Receptors in Cardio-Metabolic Diseases
Derek Strassheim, Timothy Sullivan, David C. Irwin, Evgenia Gerasimovskaya, Tim Lahm, Dwight J. Klemm, Edward C. Dempsey, Kurt R. Stenmark, Vijaya Karoor
Cells.2021; 10(12): 3347. CrossRef - PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP-1 receptor agonist-induced tachycardia in mice
Marie K. Holt, Daniel R. Cook, Daniel I. Brierley, James E. Richards, Frank Reimann, Alexander V. Gourine, Nephtali Marina, Stefan Trapp
Molecular Metabolism.2020; 39: 101024. CrossRef - A glycosylated Fc‐fused glucagon‐like peptide‐1 receptor agonist exhibits equivalent glucose lowering to but fewer gastrointestinal side effects than dulaglutide
In Bok An, Mi Sun Byun, Sang In Yang, Yuri Choi, Jung Won Woo, Hak Chul Jang, Young Chul Sung
Diabetes, Obesity and Metabolism.2020; 22(8): 1455. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists in Adult Patients With Type 2 Diabetes: Review of Cardiovascular Outcome Trials
Elodie M. Varin, Brent A. McLean, Julie A. Lovshin
Canadian Journal of Diabetes.2020; 44(1): 68. CrossRef - Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors
Alexandros Sachinidis, Dragana Nikolic, Anca Pantea Stoian, Nikolaos Papanas, Omer Tarar, Ali A. Rizvi, Manfredi Rizzo
Metabolism.2020; 111: 154343. CrossRef - GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction
Lili Zhang, Jiali Tian, Sujuan Diao, Guowei Zhang, Mochao Xiao, Dong Chang
Chemico-Biological Interactions.2020; 332: 109252. CrossRef - Predictors of Effectiveness of Glucagon-Like Peptide-1 Receptor Agonist Therapy in Patients with Type 2 Diabetes and Obesity
Alina Yu. Babenko, Daria A. Savitskaya, Yulia A. Kononova, Aleksandra Yu. Trofimova, Anna V. Simanenkova, Elena Yu. Vasilyeva, Evgeny V. Shlyakhto
Journal of Diabetes Research.2019; 2019: 1. CrossRef - Predictors of effectiveness of glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes and obesity
Ekaterina V. Tikhonenko, Alina Y. Babenko, Evgeny V. Shlyakhto
Obesity and metabolism.2019; 15(4): 22. CrossRef - Asian Subpopulations May Exhibit Greater Cardiovascular Benefit from Long-Acting Glucagon-Like Peptide 1 Receptor Agonists: A Meta-Analysis of Cardiovascular Outcome Trials
Yu Mi Kang, Yun Kyung Cho, Jiwoo Lee, Seung Eun Lee, Woo Je Lee, Joong-Yeol Park, Ye-Jee Kim, Chang Hee Jung, Michael A. Nauck
Diabetes & Metabolism Journal.2019; 43(4): 410. CrossRef - Diabetes, Incretin Therapy and Thoracic Aortic Aneurysm – What Does the Evidence Show?
Camilla Krizhanovskii , Anders Franco-Cereceda
Current Vascular Pharmacology.2019; 17(5): 432. CrossRef - Cardiovascular Effects of Different GLP-1 Receptor Agonists in Patients with Type 2 Diabetes
Gül Bahtiyar, Jean Pujals-Kury, Alan Sacerdote
Current Diabetes Reports.2018;[Epub] CrossRef - Efficacy From Strange Sources
Lawrence J. Lesko
Clinical Pharmacology & Therapeutics.2018; 103(2): 253. CrossRef - Exogenous SERP1 attenuates restenosis by restoring GLP-1 receptor activity in diabetic rats following vascular injury
Lishuai Feng, Jianbo Wang, Xu Ma
Biomedicine & Pharmacotherapy.2018; 103: 290. CrossRef - Exenatide exhibits anti‐inflammatory properties and modulates endothelial response to tumor necrosis factor α‐mediated activation
Wojciech Garczorz, Enrique Gallego‐Colon, Agnieszka Kosowska, Agnieszka Kłych‐Ratuszny, Michał Woźniak, Wiesław Marcol, K.J. Niesner, Tomasz Francuz
Cardiovascular Therapeutics.2018;[Epub] CrossRef - Molecular and clinical roles of incretin-based drugs in patients with heart failure
Bassant Orabi, Rasha Kaddoura, Amr S. Omar, Cornelia Carr, Abdulaziz Alkhulaifi
Heart Failure Reviews.2018; 23(3): 363. CrossRef - The effects of Exendin-4 on bone marrow-derived mesenchymal cells
Paola Luciani, Benedetta Fibbi, Benedetta Mazzanti, Cristiana Deledda, Lara Ballerini, Alessandra Aldinucci, Susanna Benvenuti, Riccardo Saccardi, Alessandro Peri
Endocrine.2018; 60(3): 423. CrossRef - Real-world clinical experience of Xultophy in the management of patients with type 2 diabetes in a secondary care clinic
David M. Williams, Natasha Shrikrishnapalasuriyar, Waheeba Syed, Win L. Yin, Richard Chudleigh, Stephen C. Bain, David E. Price, Jeffrey W. Stephens
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018; 12(6): 1079. CrossRef - Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome
Maja Ðanić, Bojan Stanimirov, Nebojša Pavlović, Svetlana Goločorbin-Kon, Hani Al-Salami, Karmen Stankov, Momir Mikov
Frontiers in Pharmacology.2018;[Epub] CrossRef - Cardiovascular Outcome Trials of Diabetes and Obesity Drugs: Implications for Conditional Approval and Early Phase Clinical Development
Andrew J. Krentz, Gerardo Rodriguez-Araujo
Pharmaceutical Medicine.2017; 31(6): 399. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - GLP-1R as a Target for the Treatment of Diabetic Retinopathy: Friend or Foe?
Rafael Simó, Cristina Hernández
Diabetes.2017; 66(6): 1453. CrossRef - GLP-1 receptor agonists and heart failure in diabetes
André J. Scheen
Diabetes & Metabolism.2017; 43: 2S13. CrossRef - Effects of Incretin-Based Therapies on Diabetic Microvascular Complications
Yu Mi Kang, Chang Hee Jung
Endocrinology and Metabolism.2017; 32(3): 316. CrossRef - Historique des études cardiovasculaires : de l’UGDP… aux dernières études
A.-J. Scheen
Médecine des Maladies Métaboliques.2017; 11: 2S15. CrossRef - Cardiovascular safety and benefits of GLP-1 receptor agonists
Niels B. Dalsgaard, Andreas Brønden, Tina Vilsbøll, Filip K. Knop
Expert Opinion on Drug Safety.2017; 16(3): 351. CrossRef
- Cardioprotective Actions of a Glucagon‐like Peptide‐1 Receptor Agonist on Hearts Donated After Circulatory Death

- Clinical Study
- Effects of Dipeptidyl Peptidase-4 Inhibitors on Hyperglycemia and Blood Cyclosporine Levels in Renal Transplant Patients with Diabetes: A Pilot Study
- Jaehyun Bae, Min Jung Lee, Eun Yeong Choe, Chang Hee Jung, Hye Jin Wang, Myoung Soo Kim, Yu Seun Kim, Joong-Yeol Park, Eun Seok Kang
- Endocrinol Metab. 2016;31(1):161-167. Published online March 16, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.1.161
- 5,656 View
- 58 Download
- 21 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The use of dipeptidyl peptidase-4 (DPP-4) inhibitors is increasing among renal transplant patients with diabetes. However, the glucose-lowering efficacies of various DPP-4 inhibitors and their effects on blood cyclosporine levels have not been fully investigated. We compared the glucose-lowering efficacies of DPP 4 inhibitors and evaluate their effects on the blood levels of cyclosporine in renal transplant recipients with diabetes.
Methods Sixty-five renal allograft recipients who received treatment with DPP-4 inhibitors (vildagliptin, sitagliptin, or linagliptin) following kidney transplant were enrolled. The glucose-lowering efficacies of the DPP-4 inhibitors were compared according to the changes in the hemoglobin A1c (HbA1c) levels after 3 months of treatment. Changes in the trough levels of the cyclosporine were also assessed 2 months after treatment with each DPP-4 inhibitor.
Results HbA1c significantly decreased in the linagliptin group in comparison with other DPP-4 inhibitors (vildagliptin –0.38%±1.03%, sitagliptin –0.53%±0.95%, and linagliptin –1.40±1.34;
P =0.016). Cyclosporine trough levels were significantly increased in the sitagliptin group compared with vildagliptin group (30.62±81.70 ng/mL vs. –24.22±53.54 ng/mL,P =0.036). Cyclosporine trough levels were minimally changed in patients with linagliptin.Conclusion Linagliptin demonstrates superior glucose-lowering efficacy and minimal effect on cyclosporine trough levels in comparison with other DPP-4 inhibitors in kidney transplant patients with diabetes.
-
Citations
Citations to this article as recorded by- Diabetic Kidney Disease in Post-Kidney Transplant Patients
Ngoc-Yen T. Pham, Diego Cruz, Luis Madera-Marin, Raja Ravender, Pablo Garcia
Journal of Clinical Medicine.2024; 13(3): 793. CrossRef - International consensus on post-transplantation diabetes mellitus
Adnan Sharif, Harini Chakkera, Aiko P J de Vries, Kathrin Eller, Martina Guthoff, Maria C Haller, Mads Hornum, Espen Nordheim, Alexandra Kautzky-Willer, Michael Krebs, Aleksandra Kukla, Amelie Kurnikowski, Elisabeth Schwaiger, Nuria Montero, Julio Pascual
Nephrology Dialysis Transplantation.2024; 39(3): 531. CrossRef - Metabolic Disorders in Liver Transplant Recipients: The State of the Art
Filippo Gabrielli, Lucia Golfieri, Fabio Nascimbeni, Pietro Andreone, Stefano Gitto
Journal of Clinical Medicine.2024; 13(4): 1014. CrossRef - Diabetic Kidney Disease in Post-Transplant Diabetes Mellitus: Causes, Treatment and Outcomes
Lee-Moay Lim, Jer-Ming Chang, Hung-Tien Kuo
Biomedicines.2023; 11(2): 470. CrossRef - Sweet and simple as syrup: A review and guidance for use of novel antihyperglycemic agents for post‐transplant diabetes mellitus and type 2 diabetes mellitus after kidney transplantation
S. Elise Lawrence, Mary Moss Chandran, Jeong M. Park, Helen Sweiss, Thomas Jensen, Palak Choksi, Barrett Crowther
Clinical Transplantation.2023;[Epub] CrossRef - Interventions Against Posttransplantation Diabetes: A Scientific Rationale for Treatment Hierarchy Based on Literature Review
Adnan Sharif
Transplantation.2022; 106(12): 2301. CrossRef - Dipeptidyl Peptidase-4 Inhibitor Decreases Allograft Vasculopathy Via Regulating the Functions of Endothelial Progenitor Cells in Normoglycemic Rats
Feng-Yen Lin, Chun-Min Shih, Chun-Yao Huang, Yi-Tin Tsai, Shih-Hurng Loh, Chi-Yuan Li, Cheng-Yen Lin, Yi-Wen Lin, Chien-Sung Tsai
Cardiovascular Drugs and Therapy.2021; 35(6): 1111. CrossRef - Review of Newer Antidiabetic Agents for Diabetes Management in Kidney Transplant Recipients
Sonya Anderson, Laura Cotiguala, Sarah Tischer, Jeong Mi Park, Katie McMurry
Annals of Pharmacotherapy.2021; 55(4): 496. CrossRef - Incretin based therapies and SGLT-2 inhibitors in kidney transplant recipients with diabetes: A systematic review and meta-analysis
Dora Oikonomaki, Evangelia Dounousi, Anila Duni, Stefanos Roumeliotis, Vassilios Liakopoulos
Diabetes Research and Clinical Practice.2021; 172: 108604. CrossRef - CD161a-positive natural killer (NK) cells and α-smooth muscle actin-positive myofibroblasts were upregulated by extrarenal DPP4 in a rat model of acute renal rejection
Franziska Schmid, Christina Mayer, Maike Büttner-Herold, Stephan von Hörsten, Kerstin Amann, Christoph Daniel
Diabetes Research and Clinical Practice.2021; 173: 108691. CrossRef - Current Pharmacological Intervention and Medical Management for Diabetic Kidney Transplant Recipients
Theerawut Klangjareonchai, Natsuki Eguchi, Ekamol Tantisattamo, Antoney J. Ferrey, Uttam Reddy, Donald C. Dafoe, Hirohito Ichii
Pharmaceutics.2021; 13(3): 413. CrossRef - Recent advances in new-onset diabetes mellitus after kidney transplantation
Tess Montada-Atin, G V Ramesh Prasad
World Journal of Diabetes.2021; 12(5): 541. CrossRef - Safety and Efficacy of Long-Term Administration of Dipeptidyl peptidase IV Inhibitors in Patients With New Onset Diabetes After Kidney Transplant
Adamantia Mpratsiakou, Marios Papasotiriou, Theodoros Ntrinias, Konstantinos Tsiotsios, Evangelos Papachristou, Dimitrios S. Goumenos
Experimental and Clinical Transplantation.2021; 19(5): 411. CrossRef - Medical management of metabolic and cardiovascular complications after liver transplantation
Chiara Becchetti, Melisa Dirchwolf, Vanessa Banz, Jean-François Dufour
World Journal of Gastroenterology.2020; 26(18): 2138. CrossRef - Efficacy and Safety of Dipeptidyl Peptidase-4 Inhibitors in Kidney Transplant Recipients with Post-transplant Diabetes Mellitus (PTDM)- a Systematic Review and Meta-Analysis
Tarek Samy Abdelaziz, Ahmed Yamany Ali, Moataz Fatthy
Current Diabetes Reviews.2020; 16(6): 580. CrossRef - NAFLD and liver transplantation: Disease burden, current management and future challenges
Patrizia Burra, Chiara Becchetti, Giacomo Germani
JHEP Reports.2020; 2(6): 100192. CrossRef - Linagliptin plus insulin for hyperglycemia immediately after renal transplantation: A comparative study
Rodolfo Guardado-Mendoza, David Cázares-Sánchez, María Lola Evia-Viscarra, Lilia M. Jiménez-Ceja, Edgar G. Durán-Pérez, Alberto Aguilar-García
Diabetes Research and Clinical Practice.2019; 156: 107864. CrossRef - Post-Liver Transplantation Diabetes Mellitus: A Review of Relevance and Approach to Treatment
Maria J. Peláez-Jaramillo, Allison A. Cárdenas-Mojica, Paula V. Gaete, Carlos O. Mendivil
Diabetes Therapy.2018; 9(2): 521. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Drug–drug interactions between immunosuppressants and antidiabetic drugs in the treatment of post-transplant diabetes mellitus
Thomas Vanhove, Quinten Remijsen, Dirk Kuypers, Pieter Gillard
Transplantation Reviews.2017; 31(2): 69. CrossRef - Risk assessment and management of post-transplant diabetes mellitus
Eugene Han, Myoung Soo Kim, Yu Seun Kim, Eun Seok Kang
Metabolism.2016; 65(10): 1559. CrossRef
- Diabetic Kidney Disease in Post-Kidney Transplant Patients

- Clinical Study
- Effect of Pitavastatin Treatment on ApoB-48 and Lp-PLA2 in Patients with Metabolic Syndrome: Substudy of PROspective Comparative Clinical Study Evaluating the Efficacy and Safety of PITavastatin in Patients with Metabolic Syndrome
- Hyo-Sun Lee, Chang Hee Jung, Sung Rae Kim, Hak Chul Jang, Cheol-Young Park
- Endocrinol Metab. 2016;31(1):120-126. Published online March 16, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.1.120
- 3,487 View
- 35 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Apolipoprotein (Apo) B-48 is an intestinally derived lipoprotein that is expected to be a marker for cardiovascular disease (CVD). Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a vascular-specific inflammatory marker and important risk predictor of CVD. The aim of this study was to explore the effect of pitavastatin treatment and life style modification (LSM) on ApoB-48 and Lp-PLA2 levels in metabolic syndrome (MS) patients at relatively low risk for CVD, as a sub-analysis of a previous multi-center prospective study.
Methods We enrolled 75 patients with MS from the PROPIT study and randomized them into two treatment groups: 2 mg pitavastatin daily+intensive LSM or intensive LSM only. We measured the change of lipid profiles, ApoB-48 and Lp-PLA2 for 48 weeks.
Results Total cholesterol, low density lipoprotein cholesterol, non-high density lipoprotein cholesterol, and ApoB-100/A1 ratio were significantly improved in the pitavastatin+LSM group compared to the LSM only group (
P ≤0.001). Pitavastatin+LSM did not change the level of ApoB-48 in subjects overall, but the level of ApoB-48 was significantly lower in the higher mean baseline value group of ApoB-48. The change in Lp-PLA2 was not significant after intervention in either group after treatment with pitavastatin for 1 year.Conclusion Pitavastatin treatment and LSM significantly improved lipid profiles, ApoB-100/A1 ratio, and reduced ApoB-48 levels in the higher mean baseline value group of ApoB-48, but did not significantly alter the Lp-PLA2 levels.
-
Citations
Citations to this article as recorded by- A comprehensive review on the lipid and pleiotropic effects of pitavastatin
Amirhossein Sahebkar, Nasim Kiaie, Armita Mahdavi Gorabi, Massimo R. Mannarino, Vanessa Bianconi, Tannaz Jamialahmadi, Matteo Pirro, Maciej Banach
Progress in Lipid Research.2021; 84: 101127. CrossRef - Change in ALT levels after administration of HMG‐CoA reductase inhibitors to subjects with pretreatment levels three times the upper normal limit in clinical practice
Hyunah Kim, Hyeseon Lee, Tong Min Kim, So Jung Yang, Seo Yeon Baik, Seung‐Hwan Lee, Jae‐Hyoung Cho, Hyunyong Lee, Hyeon Woo Yim, In Young Choi, Kun‐Ho Yoon, Hun‐Sung Kim
Cardiovascular Therapeutics.2018;[Epub] CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Use of Moderate‐Intensity Statins for Low‐Density Lipoprotein Cholesterol Level above 190 mg/dL at Baseline in Koreans
Hun‐Sung Kim, Hyeseon Lee, Sue Hyun Lee, Yoo Jin Jeong, Tong Min Kim, So Jung Yang, Sun Jung Baik, Hyunah Kim, Seung‐Hwan Lee, Jae Hyoung Cho, In‐Young Choi, Kun‐Ho Yoon, Ju Han Kim
Basic & Clinical Pharmacology & Toxicology.2017; 121(4): 272. CrossRef - Another statin option in HIV
Philip E Tarr, Helen Kovari
The Lancet HIV.2017; 4(7): e278. CrossRef - Clinical benefits of pitavastatin: focus on patients with diabetes or at risk of developing diabetes
Vivencio Barrios, Carlos Escobar
Future Cardiology.2016; 12(4): 449. CrossRef
- A comprehensive review on the lipid and pleiotropic effects of pitavastatin

- A Case of Malignant Pheochromocytoma Presenting as Inverted Takotsubo-Like Cardiomyopathy.
- Jung Eun Jang, Hyuk Hee Kwon, Min Jung Lee, Chang Hee Jung, Sung Jin Bae, Hong Kyu Kim, Woo Je Lee
- Endocrinol Metab. 2012;27(1):98-104. Published online March 1, 2012
- DOI: https://doi.org/10.3803/EnM.2012.27.1.98
- 1,813 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - Takotsubo cardiomyopathy or stress induced cardiomyopathy is characterized by acute transient left ventricular apical ballooning without significant coronary artery disease. The pathophysiology of Takotsubo cardiomyopathy remains unclear, but it has been suggested that the stress related neurohumoral factors, especially catecholamines, play an important role. Recently, several reports have described an inverted Takotsubo cardiomyopathy, which is characterized by the dysfunction of the basal and mid-ventricular segments sparing the apex of the heart. In this report, we present a case of a 50-year-old female with a transient left ventricular dysfunction in an inverted Takotsubo pattern, that later was diagnosed as a malignant pheochromocytoma.


 KES
KES

 First
First Prev
Prev